BUSINESS RISKS
我が国では、各都道府県において医療需要と医療機能ごとの病床の必要量を推計し地域の実情に応じた医療提供体制実現のための施策を内容とする「地域医療構想」を策定することとし、高度急性期、急性期、回復期、慢性期の4つの医療機能ごとの分化と連携を推進しております。
当社グループでは、地域における医療政策・外部環境の変化や医療機関の経営状況についてきめ細やかな情報収集に努め、ソリューションビジネスの推進による提案力の強化やスケールメリットを活かした物流効率化等、より一層地域医療への貢献を果たす施策に取り組む考えですが、医療機関における機能分化・集約が促進することで、医療機関ごとに購入する医療機器の集約が生じ販売先となる医療機関が減少する可能性、また、医療機器販売業界における競争を更に激化させる可能性があり、当社グループの販売額や収益に影響を及ぼす可能性があります。
償還価格とは、公的医療保険制度において、医療機関が診療報酬として保険機関(一部は患者の負担)に請求できる代金のうち医療材料として請求できる材料(特定保険医療材料)の請求価格であります。原則2年に1回行われる診療報酬の改定に伴い、償還価格も改定されます。特定保険医療材料の医療機関への販売価格及び仕入先からの仕入価格は、償還価格を基準にするものの、一定ではありません。また、償還価格の改定価格も各々の医療材料により全て異なります。従って、償還価格の改定による販売額や収益への影響額を事前に算定することは困難であります。
当社グループにおいては、このような償還価格の対象となる特定保険医療材料の販売高が全体の3分の1程度を占めており、償還価格の改定が当社グループの販売価格や売上総利益率の低下傾向に作用する場合には、当社グループの販売額や収益に影響を及ぼす可能性があります。
当社グループでは、変化する業界環境に対応して成長を維持し、多様化する医療現場のニーズに応えるため、中長期的な経営戦略として、各地域に密着した企業とのM&Aによる企業規模の拡大を目指しています。スケールメリットを活用したコスト削減や業務効率化により、安定的な成長と企業価値の向上を図る考えです。
しかしながら、医療機器販売業界は中小規模の企業が多く、そのほとんどが非上場企業であり、必ずしも企業価値算定の基準となる市場価格が存在するわけではなく、財務内容の精緻化及び透明性においても十分ではないものと認識しております。当社グループでは、取得価格や合併比率等の決定にあたっては、事前調査を実施の上で財務状況や事業計画の進捗状況、将来の見通し等を総合的に勘案し、規模等に応じ独立した第三者算定機関による企業価値算定結果をも踏まえた上で、可能な限り慎重に交渉・協議する考えですが、根拠とした事業計画を達成できる保証は無く、結果として予測どおりの収益を得られないと判断された場合には、「のれん」の減損損失を計上する可能性があり、これにより当社グループの収益に影響を及ぼす可能性があります。また、事前調査にあたっては、細心の注意を払い可能な限り正確に実施する考えですが、買収・合併後に簿外債務やコンプライアンス上の問題が発生する可能性があり、これにより当社グループの収益に影響を及ぼす可能性があります。
また、M&Aの対象となる各社にはそれぞれの企業文化と従業員がいることを認識しております。当社グループでは、地域に密着した各社の企業文化と従業員を尊重し、グループとして手を携えていく考えですが、企業文化の融合や人事交流が円滑に実施できず、人材が流出してしまう場合や基幹システム・業務手順の統合が徹底できない場合には、M&Aによる業務の効率化やシナジー効果等の予測された効果が発揮できない可能性があり、これにより当社グループの販売額や収益に影響を及ぼす可能性があります。
当社グループでは、多様化する医療現場のニーズに応えるため、ソリューションビジネスの推進による提案力の強化やスケールメリットを活かした物流効率化等、より一層、地域医療への貢献を果たす施策に取り組み、企業価値の向上に努めていく考えです。当社グループが新規事業に取り組む場合には、事前に十分な検討を行った上で事業計画が策定され、取締役会における承認の上で行われます。しかしながら、新規事業の展開には先行投資が必要となるケースが多く、当該事業が安定して収益を計上するまでには相当の時間を要することが予想されるため、一時的に当社グループの利益率が低下する可能性があります。また、医療業界の環境変化等により当該事業が当初の事業計画どおりに展開できなかった場合には、投資を回収できなくなる可能性があり、当社グループの収益に影響を及ぼす可能性があります。
当社グループは、購買から販売、請求・入金といった各業務を連携・統合する基幹システムをグループ事業会社に導入することで効率的な経営体質と内部統制の強化を図っており、各部が連携し、運用する営業現場や管理部門からの情報・意見を汲み上げながら、今後も、システムの機能強化や更なる整備に取り組み、より付加価値の高いシステム環境を構築していく方針です。しかしながら、システム環境の構築には多額の設備投資が必要となる一方で、医療現場の運用や多様化するニーズとの間に齟齬が生じてしまった場合、新規運用についての成熟が思うように進まなかった場合には、かえって営業生産性や業務効率性を低下させる可能性があり、これにより投資を回収できなくなる可能性、当社グループの販売額や収益に影響を及ぼす可能性があります。
当社グループは事業の遂行にあたって、以下のような法的規制の適用を受けております。
そのため当社グループでは、医療に携わる企業として、「正義と利益のどちらかを取らなければならない状況に遭遇したら、迷わず正義を取れ」を企業活動の基本姿勢とし、コンプライアンスガイドラインの策定、eラーニングでの社内研修制度により、当社グル―プの役員及び従業員としての行動規範の周知徹底を図り、法的規制に対する違反行為のリスクを低減するよう努めています。また、他の業務執行部門から独立した当社の内部監査室による内部監査を実施し、内部管理体制の適切性・有効性を検証、評価し、その改善を促すことにより、法令を遵守するための体制構築に取り組んでおります。しかしながら、これらの対策を行ったとしても、役員及び従業員による個人的な不正行為を含む法的規制に対する違反行為のリスクを回避できない可能性があります。法的規制に対する違反行為があった場合には、違反の内容に応じて、許認可等の取消その他の行政処分、罰金刑といった法的制裁を受ける可能性の他、取引先からの取引停止を受ける可能性、当社グループへの信頼低下等による販売活動へ影響が生じる可能性、被害者に生じた損害の賠償、内部管理体制の改善・強化等のために多額の費用が生じる可能性があり、これにより当社グループの販売額や収益に影響を及ぼす可能性があります。
医療機器の販売業・貸与業・修理業・製造販売業、医薬品及び再生医療等製品の販売業について医薬品医療機器等法による規制の適用を受けており、その他遂行する事業、取扱う商品・サービスに応じて、毒物及び劇物取締法、介護保険法、建設業法といった各種業法による規制の適用を受けております。医薬品医療機器等法を含む各種業法に基づき取得している主な許認可等については、次のとおりです。
| 対象 | 法令等名 | 法的規制の内容 |
|---|---|---|
| 高度管理医療機器等 販売業・貸与業 |
医薬品 医療機器等法 |
医薬品医療機器等法第39条第1項の規定により 許可を受けております。 |
| 動物用高度管理医療機器等 販売業・貸与業 |
医薬品 医療機器等法 |
医薬品医療機器等法第39条第1項の規定により 許可を受けております。 |
| 医療機器修理業 | 医薬品 医療機器等法 |
医薬品医療機器等法第40条の2第1項の規定により 許可を受けております。 |
| 第二種 医療機器製造販売業 |
医薬品 医療機器等法 |
医薬品医療機器等法第23条の2第1項の規定により 許可を受けております。 |
| 医薬品販売業 | 医薬品 医療機器等法 |
医薬品医療機器等法第24条第1項の規定により 卸売販売業の許可を受けております。 |
| 再生医療等製品販売業 | 医薬品 医療機器等法 |
医薬品医療機器等法第40条の5第1項の規定により 許可を受けております。 |
| 動物用医薬品販売業 | 医薬品 医療機器等法 |
医薬品医療機器等法第24条第1項の規定により 卸売販売業の許可を受けております。 |
| 毒物劇物販売業 | 毒物及び 劇物取締法 |
毒物及び劇物取締法第4条第1項の規定により 一般販売業の登録を受けております。 |
| 福祉用具販売事業 | 介護保険法 | 介護保険法第70条第1項及び第115条の2第1項の規定により 指定特定福祉用具販売事業者及び指定特定介護予防福祉用具販売事業者の指定を受けております。 |
| 福祉用具貸与事業 | 介護保険法 | 介護保険法第70条第1項及び第115条の2第1項の規定により 指定福祉用具貸与事業者及び指定介護予防福祉用具貸与事業者の指定を受けております。 |
| 一般建設業 | 建設業法 | 建設業法第3条第1項の規定により 一般建設業の許可を受けております。 |
当社グループの販売先には国公立病院等の公的な医療機関が含まれており、取引にあたっては入札が実施されることもあるため、贈賄防止に関する法令や入札談合を禁止する独占禁止法を遵守する必要があります。なお、当社グループは米国メーカーの医療機器を多数取り扱っており、贈賄防止に関する法令については国内法だけでなく、米国海外腐敗行為防止法(FCPA)等の国外法にも注意を払う必要があります。
景品表示法は医療機器販売業を含む医療機器業等の業種に適用する特別の景品規制を設けており、当社グループは医療機関等に対して、医療機器の取引を不当に誘引する手段として、医療機器の使用のために必要な物品又はサービスその他正常な商慣習に照らして適当と認められる範囲を超えて景品類を提供することを禁止されております。景品規制については、同法の規制に加え、当社グループが属する業界の自主規制団体である医療機器業公正取引協議会が制定した医療機器業公正競争規約についても遵守する必要があります。
当社グループでは従業員の個人情報の他、医療機関が保有する個人情報、医療機器・介護福祉機器の個人販売先の個人情報を取扱うことがあります。個人情報を取扱うにあたっては、個人情報保護法に基づき、適正な取得や漏えい防止のための管理体制を整備する必要があります。
当社グループは、首都圏をはじめとする各地に拠点を置き、広範囲に事業活動を展開しております。地震、火災、台風、洪水、雪害等の自然災害の発生に備え、事業継続計画(BCP)を策定し、当社グループも医療業界の一員として医療インフラの継続を図るための安定供給体制の整備に努めております。災害の発生に備え、神奈川県内に免震構造の物流センターを、群馬県内に倉庫面積19,000㎡超の大規模物流センターを有し、商品供給維持のためのバックアップ体制の拡充に努めております。しかしながら、当社グループの事業範囲は広範囲であり、昨今の気候変動に伴う災害の大規模化を鑑みると、災害が発生した場合のリスクを全て回避することは困難であります。災害の規模が想定を大きく上回り、当社グループの本社・事業拠点、倉庫施設等の被災により商品が汚損・破損した場合、従業員の勤務が困難となった場合、流通経路の寸断により納品が困難となった場合、顧客及び仕入先等の被災により販売及び仕入が困難となった場合には、経常的な事業運営に支障をきたし、当社グループの販売額や収益に影響を及ぼす可能性があります。
当社グループは、病院や診療所等の医療機関と日常的に密接な関わりを持ち事業活動を行っております。当社グループは、医療関係者として医療機関に準じた感染予防対策を含んだ新興感染症BCPマニュアルを策定し、従業員・顧客・取引先の安全対策の実施に努めております。しかしながら、新型インフルエンザや新型コロナウイルス感染症のような大規模な新興感染症が発生し、感染拡大の規模やスピードが想定を大きく上回った場合には、一時的な事業停止、仕入の遅滞、在庫の滞留、売掛債権回収の遅延等経常的な事業運営に支障をきたし、当社グループの販売額や収益に影響を及ぼす可能性があります。
医療機器や医薬品をはじめとして、当社グループで取扱う製品の一部には、製造元により使用期限が設定されています。当社グループでは、より安全で高品質な製品を医療・介護福祉の現場にお届けすることを目指し、定期的な実地棚卸の実施その他運用の徹底・検証、ITシステムの活用により使用期限管理体制の改善・強化に取り組んでおります。しかしながら、万が一、当社グループの人為的要因やシステムトラブルにより使用期限を経過した製品が流通し重大な健康被害が生じた場合には、医療機器販売業等に係る許認可等の取り消し、当社グループへの信頼低下等により販売活動へ影響が生じる可能性や、患者様・医療機関等への補償、使用期限管理体制の改善・強化等のために多額の費用が生じる可能性があり、これにより当社グループの販売額や収益に影響を及ぼす可能性があります。
当社グループでは、医療機関のニーズを重視したプライベートブランド商品の販売を行っておりますが、医療関連製品であることから、確かな品質を追求しております。ディーラーからメーカーへと立場を変え、責任ある商品の選定・供給に努めておりますが、プライベートブランド商品に予期しがたい欠陥や不具合が発生した場合には、商品回収や損害賠償等による多大な費用負担に加え、当社グループへの信頼低下により、当社グループの販売額や収益に影響を及ぼす可能性があります。
医療技術は日々進歩しており、例えば心疾患治療における低侵襲性医療の発展により、使用される医療機器にも変化があります。当社グループは、医療機器の総合ディーラーとして、特定の領域に偏ることなくほぼ全ての領域の医療機器を取扱っておりますが、今後の医療技術の革新により、取扱っている医療機器の使用が減少する場合には、当社グループの販売額や収益に影響を及ぼす可能性があります。
当社グループでは、取引先の現状、将来性、経営者、業界事情等を評価・判断し与信管理規程に則った取引先別の与信限度額を設定し、与信管理を徹底することで、貸倒れ等を未然に防止し、且つ最小限に抑えるよう努めております。しかしながら、取引先の業績悪化等で予期せぬ貸倒れリスクが顕在化し、損失・引当の計上が必要となった場合には、当社グループの収益に影響を及ぼす可能性があります。
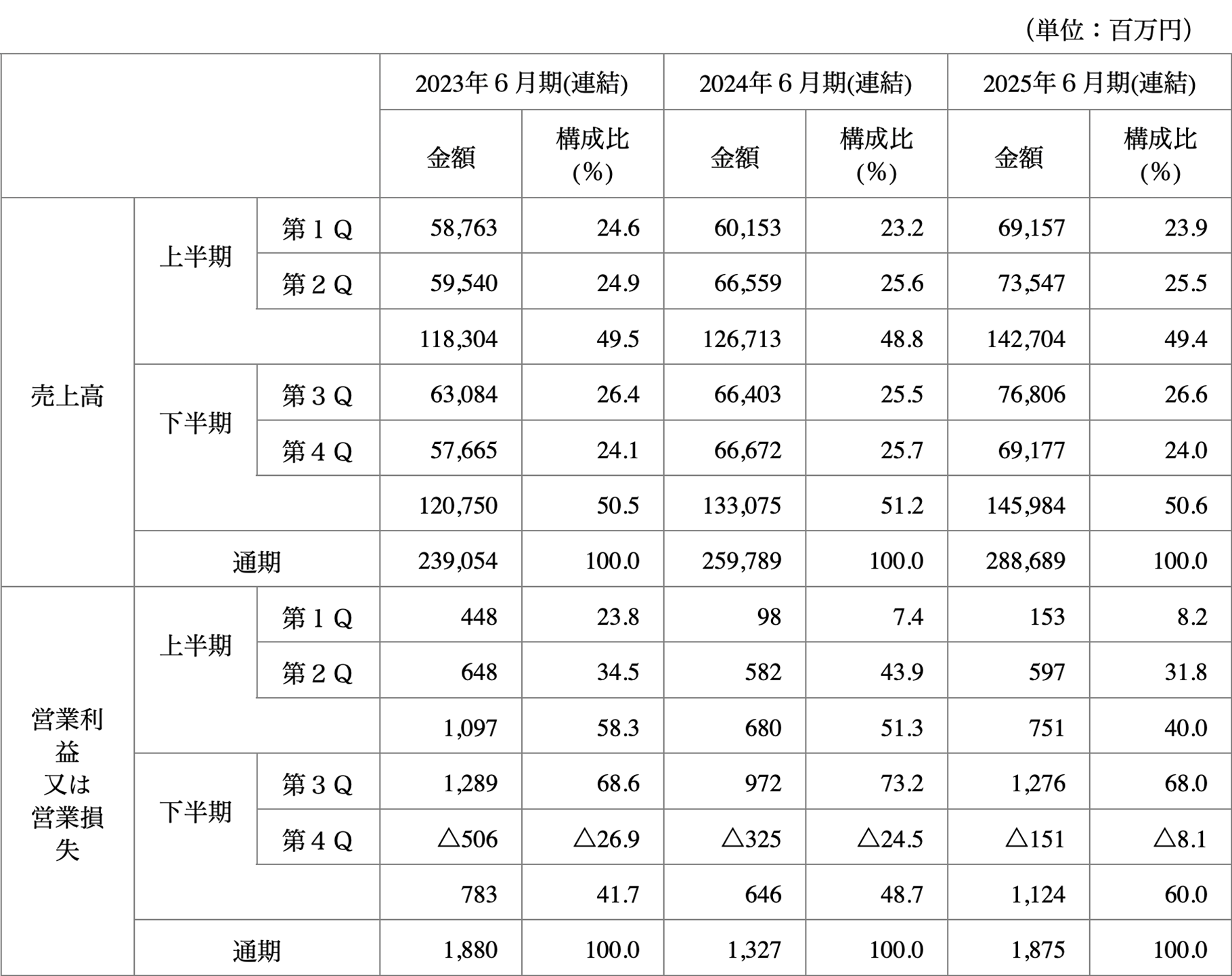
当社グループの販売先には国公立病院等の公的な医療機関が含まれており、当該医療機関は12月及び年度末である3月において設備投資を集中して行う傾向があるため当社グループの販売高は毎年第2Q及び第3Qにおいて他の期より高くなり、これに連動して利益も当該時期に増加する傾向があります。また、その反動で第4Qにかけての販売高が他の期より低くなり、これに連動して利益も当該時期に減少する傾向があります。また、医療機関の新築、移転、増築が行われる際には、多額の医療機器の一括購入が発生し、一時的に販売高が増加する場合があります。従って、当社グループの四半期の経営成績は、通期の経営成績に連動するものではなく、四半期又は半期の経営成績だけをもって、通期の経営成績を予想することは困難であります。
なお、2023年6月期から2025年6月期における各四半期の売上高及び営業利益又は営業損失の状況は、以下のとおりであります。