The "treatment app" is attracting attention as the third treatment following pharmaceuticals and medical devices. The smartphone app supports the treatment blank period between visits and enhances the therapeutic effect. In the United States, which is ahead, there are examples such as support for the treatment of diabetes and lung cancer, and some have already been approved by the FDA (US Food and Drug Administration). In Japan as well, the development of treatment apps is being promoted mainly by venture companies. Treatment apps are attracting attention as a means of treatment for diseases for which treatment results have been insufficient with conventional treatments, and are also expected from the perspective of curbing national medical expenses, which are increasing year by year.

Lifestyle-related diseases do not show clear symptoms, and patients lack insight, and it is said that there is a certain limit to treatment with medication alone. It is said that less than 30% of people with nicotine addiction can continue to quit smoking, and more than 50% of people with diabetes continue to quit smoking. Therefore, the treatment app has emerged as a cover for the limitations of conventional treatment methods.
The treatment method using the app is different from the treatment method using conventional medicines and medical devices, and incorporates the smartphone owned by the patient as part of the treatment. Unlike ordinary apps, it is installed and used on the patient's smartphone according to the doctor's prescription. The Pharmaceuticals and Medical Devices Act (Pharmaceutical Machinery Act) of 2014 was amended to allow software to be treated as a "medical device program".
As for the mechanism of the treatment app, when the patient inputs information such as physical condition, the algorithm built based on the latest medical evidence built into the app analyzes and provides the patient with individually optimized medical advice at the appropriate time. We will provide it and support patients diligently until the next visit. In other words, the aim is to fill the blank treatment period between visits with appropriate follow-up by the app and maintain the willingness to continue treatment.
Applicable diseases include diabetes, nicotine addiction, depression, chronic heart failure, fatty liver, alcoholism, cancer, Parkinson's disease, asthma, COPD, chronic kidney disease, hypertension, dyslipidemia, etc. Development is in progress (table).
In the United States, FDA-approved and insurance-approved diabetes and cancer treatment apps are already being used by patients in clinical practice as “medical devices”.
The type 2 diabetes treatment app "BlueStar" developed by WellDoc in the United States in collaboration with the American Diabetes Educator Association is a type 2 diabetes treatment app that allows patients to enter blood glucose levels, medication status, physical condition, etc., and the app analyzes the information and informs the patient. Coaching to improve lifestyle and exercise habits and maintain motivation at the right time. You can also ask a specialist on the app. The patient's input information is sent to the medical team including the attending physician and the information is shared.
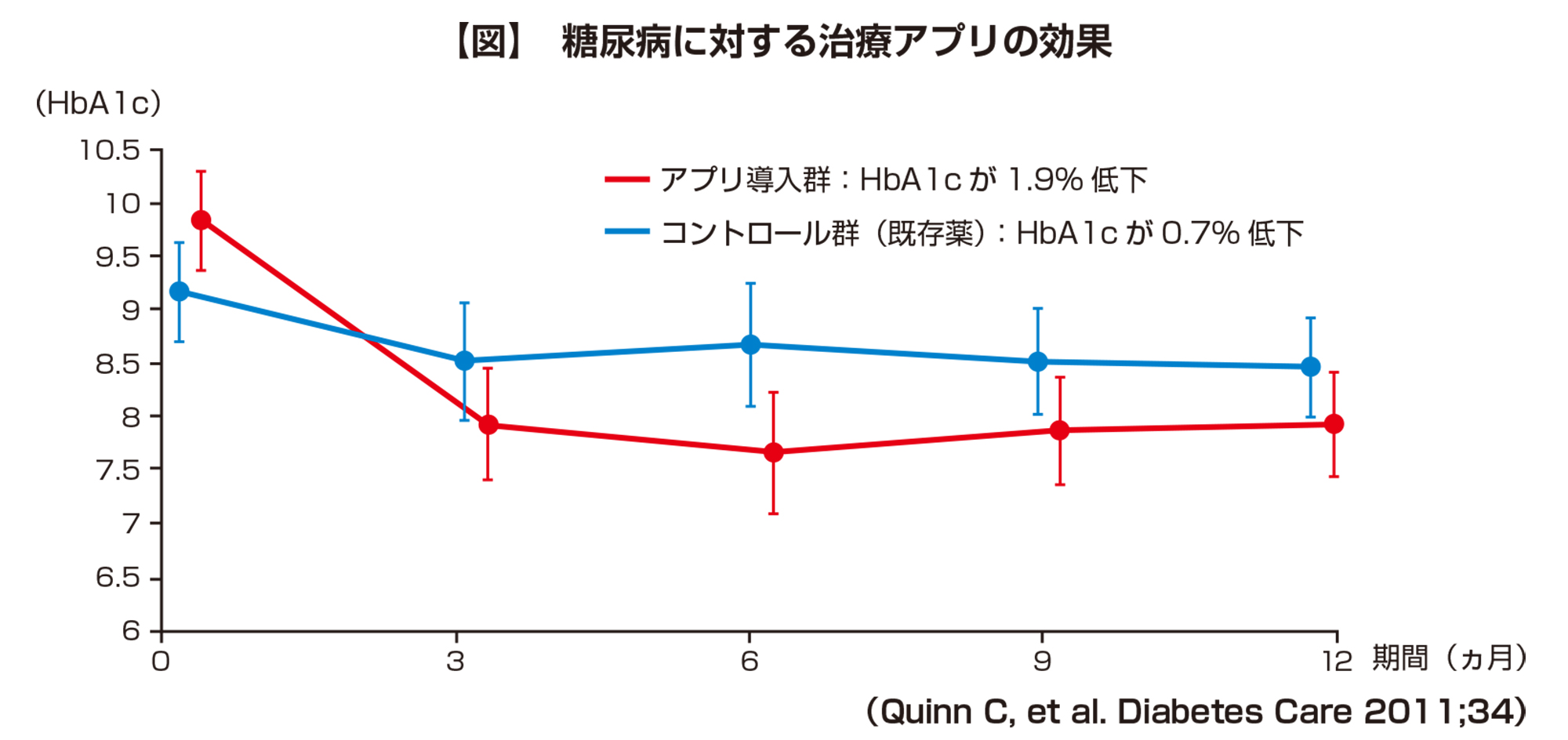
According to the clinical trial data, in a multicenter cluster randomized controlled trial of 163 diabetic patients, the HbA1c level decreased by 0.7% in the control group (existing drug) during the 1-year observation period. The app-introduced group was significantly better with a 1.9% decrease. In other words, it was demonstrated that the app treatment has a therapeutic effect equal to or better than that of existing drugs (Fig.). BlueStar was approved by the FDA in 2010 and is covered by several major insurance companies. The average health care cost for diabetics in the United States is estimated to be $ 13,000- $ 20,000 / year, but BlueStar has been shown to save $ 3,000 / year in health care per patient. , The effect of decrease in the number of doses and dosage due to compliance with medication and improvement of lifestyle was considered.
In addition, the lung cancer treatment app "Moovcare" developed by Sivan Innovation of Israel allows patients to enter their 12 symptoms every week, and the app analyzes the symptoms and reports the results to the attending physician. Evaluate changes in symptoms according to an algorithm, and if there are specific changes in the patient's condition, notify the attending physician by e-mail and encourage them to consider examinations and consultations. Phase 3 clinical trial data presented by the research group at the Jean Bernard Cancer Institute in France at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting show that it is a multicenter study of 133 patients with advanced lung cancer. In a joint randomized controlled design clinical trial, median overall survival was extended by 7 months compared to 19 months in the app-introduced group and 12 months in the control group (standard follow-up). The quality of life (QOL) was also better in the app-introduced group.
Apple Inc. has developed an app "Apple Heart Study" to detect heart abnormalities in collaboration with Stanford University. The sensor built into the Apple Watch is used to measure data such as wrist blood flow and heart rate, and the data is analyzed by a unique algorithm to detect atrial fibrillation, which is a type of arrhythmia, and notify the user. To do. Apple Inc. began collecting data for the Apple Heart Study in February this year, revealing that its purpose is to approve clinical trial equipment from the FDA.
Treatment apps in the United States are also used as remote guidance by doctors.
The number of research papers on app mobile health was about 100 in 2004, but exceeded 800 in 2010, and it is now published in major medical journals such as The Lancet and The New England Journal of Medicine. It's coming.
In Japan, for diagnostic support, insurance was already applied in April 2016 to the diagnostic imaging device application "Join" that supports the decision of t-PA administration for cerebral infarction, but the therapeutic effect on patients. There is no practical application for singing. The leading development is an app named "CureApp Smoking Cessation" (co-developed by CureApp and Keio University) that supports smoking cessation treatment. The app not only records smoking status, smoking urges, medication status, etc., but also gives medically valid advice similar to doctor's guidance based on the data. Since last fall, clinical trials have been conducted at 20 to 30 medical institutions, including Keio University Hospital and Saitama Municipal Hospital. After three months of treatment, the success rate of smoking cessation in the group using the treatment app was about 30% higher than that in the group not using it. In addition, an app "GlucoNote" (co-developed by DoCoMo and the University of Tokyo) that supports the treatment of type 2 diabetes has also started clinical trials.
The treatment app is expected to have the same or better therapeutic effect at a lower cost than conventional medical products. The development cost of one drug is said to be about 280 billion yen including failure cases, which leads to expensive drug prices and puts pressure on medical finances. On the other hand, the development cost of the treatment application is said to be about several hundred million yen, which is much cheaper, and the drug price is expected to be low. It is also expected to be a means to stop the rise in medical expenses. Although there are differences depending on the medical institution, it is said that the high retention rate of the app will be maintained, especially for doctors who are enthusiastic about management guidance.
Treatment apps developed in Japan include those for nicotine addiction and diabetes, as well as NASH (non-alcoholic steatohepatitis) and insomnia.