Ministry of Health, Labor and Welfare formulates cleaning guidelines for remanufacturing by next spring
Recently, the Ministry of Health, Labor and Welfare announced that it will formulate cleaning guidelines for remanufacturing used single-use devices (SUDs) by spring 2019. The ministry revised the ministerial ordinance in July 2017 and introduced a system that allows reuse if the SUD is properly remanufactured, but cleaning and sterility are essential in determining its suitability. In this announcement by the Ministry of Health, Labor and Welfare, the aim is to create an environment where many businesses can easily enter and to activate the movement toward remanufacturing of SUD by concretely showing the guideline for cleaning and sterility. There seems to be one.
Reuse of used SUD has been prohibited in Japan in principle. The June 2014 Notice of the Medical Affairs Bureau entitled "Re-dissemination of the handling of SUD, etc." also states that "SUD will not be used unless there is a specific reasonable reason." However, in reality, many medical institutions reuse SUDs, and there were many cases where product deterioration, incomplete cleaning and sterility led to medical accidents and nosocomial infections.
One of the reasons why reuse of SUD is not eliminated is that SUD is expensive. Some SUDs contain rare metals, and it is not uncommon for a single SUD to cost hundreds of thousands of yen. As a result, there was no end to the voices of medical professionals saying, "It is a waste to dispose of SUD in a single use."
If the SUD can be reused with sufficient safety, it will lead to a reduction in medical costs. However, if safety decisions are left to individual medical institutions, it is not possible to guarantee sufficient safety. Then what should we do. This would require the country to develop and institutionalize guidelines for the reuse of SUDs. It was the revision of the ministerial ordinance in July 2017 that was made from such an idea.
The problem of medical institutions self-judging the safety of SUD and reusing it has frequently occurred overseas. Therefore, in the United States, in 2000, the US Food and Drug Administration (FDA) set out safety standards for the reuse of SUD and institutionalized them. After the used SUD has been collected, disassembled, cleaned, replaced, reassembled, sterilized, etc., it is newly approved by the FDA as a remanufactured SUD separate from the original (new) product. Remanufactured SUDs are not only returned to the medical institution that collected the used SUD, but also sold to a completely different medical institution. This can be said to be an open model system. In the United States, which precedes it, remanufactured SUDs are distributed in the price range of about 50 to 70% of the original products, and are said to be an important option for controlling medical expenses.
In Germany, a remanufacturing SUD system has been introduced since 2002. Remanufactured products are treated as equivalent to the original products under the conditions that meet the "Recommendations for Hospital Hygiene and Infection Prevention" (KRINKO Recommendations) by the Commission of the Robert Koch Institute and the Federal Institute for Pharmaceuticals and Medical Devices. Individual medical institutions contract with the remanufacturer, and the remanufactured SUD is delivered to the original medical institution. Certification as a medical device (CE mark) is not required (however, certification as a medical device is required if it is widely distributed in the market, not in a specific hospital). This is a closed model system. It is subject to remanufacturing regardless of single-use products, multiple-use products, and material classification. Since the KRINKO recommendation is issued to both hospitals and remanufacturing companies, hospitals can remanufacture SUDs if they meet this recommendation, but since this recommendation is extremely strict, hospitals use remanufacturing companies. You have chosen that. Currently, it is said that about 90% of university hospitals use SUD remanufactured products.
In the UK, remanufactured products require the CE mark and must be distributed only between specific hospitals and remanufacturers. In other words, it can be said to be a hybrid type of the US model and the German model.
In the European Union (EU) countries except France, unified regulations were established in 2017, and the movement of remanufacturing SUD is becoming active.
In France, there is currently no active promotion of remanufactured SUDs, probably due to the effects of Creutzfeldt-Jakob disease caused by the reuse of SUDs.
The following three points are the points of Japan's remanufacturing SUD system, which was indicated by the revision of the ministerial ordinance in July 2017. (1) In order to manufacture and sell remanufactured SUD, a manufacturing and sales license based on the Pharmaceutical and Medical Equipment Law is required. (2) Remanufactured SUD is a separate item from the original product and requires manufacturing and marketing approval. (3) Remanufactured. Responsibility under the Pharmaceutical and Medical Device Law (safety measures, collection, etc.) related to SUD is borne by the manufacturer and distributor who remanufactured.
With the implementation and operation of the remanufacturing SUD system, new standards regarding the quality and manufacturing control of remanufactured SUDs have been established, and "they must be properly controlled by domestic medical institutions" and "contamination and pathogens are manufactured." It was decided that it should be removed and inactivated in the process, and that the structure of the original product, changes in raw materials, and monitoring of safety information should be made. In addition, in order to ensure the traceability of the remanufactured SUD, it is also stipulated that the remanufactured SUD should be given a serial number and information should be managed from the medical institution that collected the used SUD to the manufacturing process and distribution. (Figure).
Target products for remanufactured SUD include neurophysiological electrode catheters (EP catheters), trocars, and vascular sealing devices for laparoscopic devices, which have already been targeted in Europe and the United States. Those that come into contact with the brain, spinal cord, dura mater, cranial ganglion, etc. are excluded because of the high risk of infection.
Following the establishment of a new recycling system for single-use medical devices by the Ministry of Health, Labor and Welfare, nine companies aiming to create new industries (Aitec, Olympus, Sakura Global Holdings, Sakura Seiki, Johnson & Johnson, Daiichi Medical Co., Ltd., in January this year, Japan Striker, Hogy Medical, MEDIUS Solutions) has launched a single-use medical device remanufacturing promotion council. While ensuring medical safety, it aims to make effective use of resources and reduce medical costs.
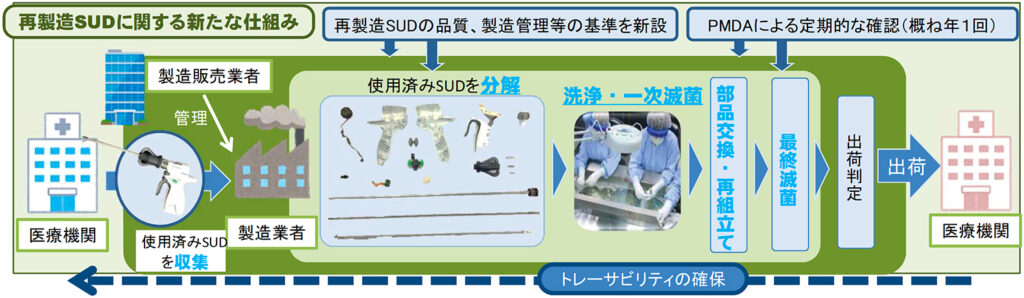 Figure Remanufacturing of single-use device (SUD)
Figure Remanufacturing of single-use device (SUD)
source: Ministry of Health, Labor and Welfare website
With the introduction of remanufactured SUDs, first of all, it is expected that infection accidents due to SUD reuse at the self-judgment of medical institutions will be greatly reduced. If there is a problem with the remanufacturer, there is a risk that an accident will occur through it, but to date, no accidents due to remanufacturing SUD have been reported in the United States, which is ahead of the others.
It will also lead to a reduction in medical costs. According to industry insiders, the market size of SUD in Japan is about 1.5 trillion yen, of which about 10% is suitable for remanufacturing. The price of the remanufactured SUD is expected to be 50-70% of the original product. Therefore, it is estimated that the introduction of remanufactured SUD will reduce medical costs by 75 to 45 billion yen.
After the revision of the ministerial ordinance in July 2017, the movement to promote remanufacturing SUD has become active in various fields.
In January 2018, a voluntary organization "Single Medical Device Remanufacturing Promotion Council" was formed centered on remanufacturing SUDs and companies aiming to enter related businesses. The council intends to actively work on coordinating opinions with related ministries and agencies, making recommendations, and examining technical issues in order to popularize remanufactured SUDs.
The revision of the ministerial ordinance states that the cleaning and sterility of remanufactured SUDs will be performed in accordance with the existing guidelines for cleaning and sterility of medical devices that can be used multiple times. However, the risk of infection differs between SUD and medical devices that can be used multiple times, and the risk of infection differs depending on the product of SUD. It is doubtful that this will be uniformly cleaned and sterilized according to the same standards as existing medical devices that can be used multiple times. Therefore, the Ministry of Health, Labor and Welfare has announced that it will formulate guidelines for cleaning when remanufacturing new SUDs by the spring of 2019. The details of the guidelines are still unknown, but in any case, it is thought that this will promote more detailed cleaning and sterility according to the degree of SUD infection risk, product material and degree of deterioration.
Cleaning and sterility are the key to promoting the spread of remanufactured SUDs, and the lack of clear guidelines may be a barrier for businesses to hesitate to enter. It is believed that the Ministry of Health, Labor and Welfare's announcement is intended to encourage more businesses to enter the market by removing this concern. Whether the movement toward remanufactured SUD will become more active, the outcome will be watched.