Japan's largest medical material database.
With high completeness and reliability,
Contributes to information gathering and improvement of business efficiency.
Japan's largest medical material database built by MEDIUS Holdings.
We create a database of medical material information with high completeness, reliability, and immediacy, and contribute to information collection and improvement of work efficiency.
What is required of the medical material database
01
Comprehensiveness of registered product information
02
Prompt maintenance and reliability of registered product information
Medical materials used in medical institutions range from highly specialized medical equipment to sanitary daily necessities such as bandages.
In the medical material database, the higher the comprehensiveness, the better.
This will lead to a reduction in the burden of medical material research work at medical institutions and wholesalers of medical materials and an improvement in accuracy.
While new products are emerging in medical materials, some are discontinued.
In addition, the insurance reimbursement price and sales price will change, so maintenance of the information registered in the medical materials database requires immediacy and reliability.
ASOURCE ® DATABASE
Overview and features of for MEDICAL DEVICE
In Japan, there is no official database on medical materials, and there are organizations and companies that create their own.
It does not cover all medical material information.
MEDIUS Holdings, we aim to improve efficiency and prevent mistakes in the management of medical materials at medical institutions and other companies.
ASOURCE ® DATABASE for MEDICAL DEVICE (hereinafter referred to as ASOURCE) ® DATABASE) is provided.
01
Comprehensiveness of registered product information
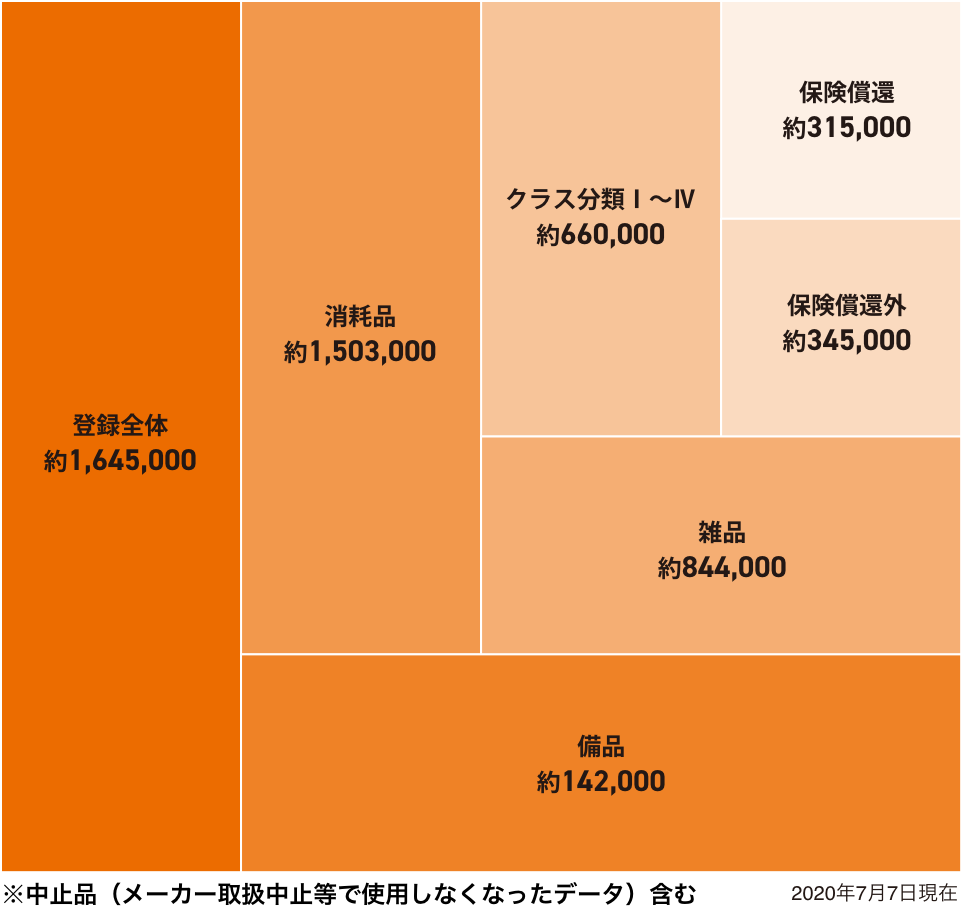
ASOURCE ® The number of DATABASE registrations has reached approximately 1.65 million at this time (as of the end of July 2020).
The data items registered for each medical material are the product name, manufacturing company name, and LOT / serial management classification, Global Trade Item Number (GTIN), JAN Code (Multiple codes can be registered per product), List price history, Redemption price history, Safety Data Sheet (SDS) is. Items shown in red are A SOURCE ® It is unique to DATABASE and is not included in the management items of other companies' medical material databases.
Figure 1: Number of ASOURCE ® DATABASE registrations
02
Of the registered product information
With quick maintenance
Ensuring reliability
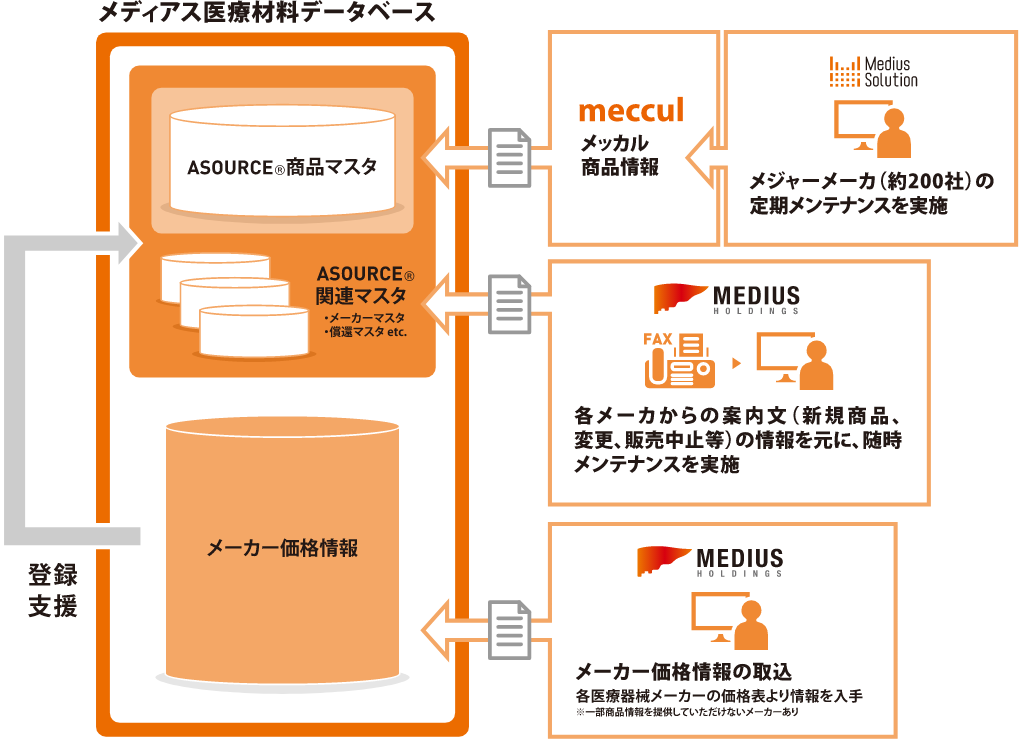
The data in ASOURCE ® DATABASE is based on information from the meccul medical material database and each medical material manufacturer (Fig. 2). In addition to our own information gathering, by receiving information provided in close cooperation with each manufacturer, we can quickly register and change information and improve its reliability.
Figure 2: Utilization of multiple product information acquisition routes
how to use
After applying for use from the dedicated page and obtaining an ID and password, you will be able to search for medical materials and browse information free of charge.
Note) The official name of ASOURCE DATABASE is ASOURCE ® DATABASE for MEDICAL DEVICE.
