ASOURCE®NAVI

公開日:2021.12.17
新型コロナウイルス感染症の流行によって対面せずに診療する「オンライン診療」に関心が集まりましたが、通院時以外の行動の変化を引き起こすことで治療効果を高めるデジタル治療用アプリもニューノーマル時代の新たな治療選択肢となる可能性があります。デジタル治療用とは、従来治療がターゲットしてこなかった患者の行動変容に働きかける治療法で、医薬品や医療機器と同様に、臨床試験によるエビデンスの創出を通じて承認・保険償還のプロセスを経て効果が実証された医療機器を指します。医薬品、医療機器に次ぐ第三の治療ともいわれています。患者に対する治療効果の個別最適化や重症化予防、医師の業務負担の軽減などに寄与するものと考えられています。仕組みとしては、医師の処方によりアプリを患者のスマートフォンやタブレット端末にインストール(ダウンロード)して使用。患者が体調などの情報を入力すると、アプリに組み込まれたアルゴリズムが解析し、最適なタイミングで個別化された診療アドバイスを患者に提供し、次回の受診までの期間、細やかなサポートを行い、治療継続意欲を持続させます。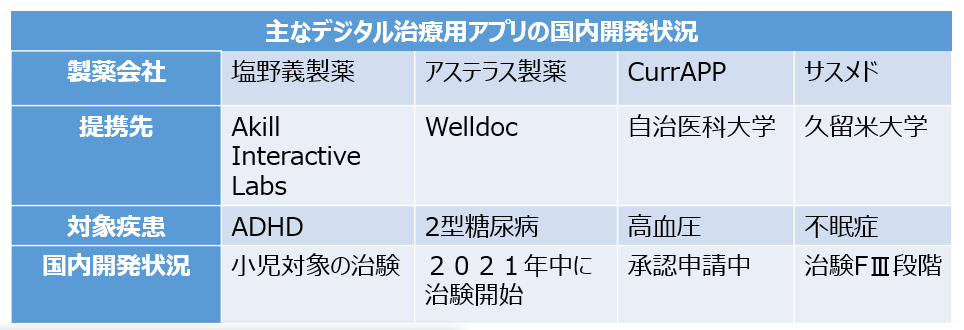
デジタル治療用アプリとして最も早く開発されたのは米国のWelldoc社が開発した糖尿病患者向けアプリBlueStarです。アメリカ食品医薬品局(FDA)が2010年に医療機器として承認しており、米国とカナダで販売されています。BlueStarは開発費が医薬品の約10分の1と少なく、治験でのHbA1cの低下効果は1.2%(既存薬との併用)と、新薬の効果を上回っていました。糖尿病患者が日々の血糖値をアプリに入力すると、個々人に応じた食事指導や運動を促すメッセージ、適切な投与インスリンの量等の情報をアプリから受け取ることができます。日本では、アステラス製薬が同社と提携し、開発を進めることにしています。
その他、小児の注意欠如・多動症(ADHD)における不注意症状の改善を適応としたデジタル治療用アプリ「AKL-T01」がアメリカ食品医薬品局(FDA)から承認を受け、欧州でもCEマークを取得しています。Akill Interactive Labsが開発したもので、8〜12歳の小児のADHDに対して、アプリを用いて不注意症状を改善します。認知機能で重要な役割を果たす脳の前頭前野を活性化するように設計されており、患者はスマホやタブレット端末を通じて、個別に最適化された難易度のゲームを継続的に行うことで、症状が改善されます。臨床試験では注意および抑制制御に関する客観的な評価方法の注意機能スコアを用い、Attention Performance Indexの変化量を調べたところ、アプリ使用群は対照群(ワードパズル)に対して統計学的に有意な改善を認めました。日本では、塩野義製薬がAkill Interactive Labsから「AKL-T01」の販売権を得て、臨床試験を進めています。
ニコチン依存症に対する日本初の治療用アプリ「CureApp SC ニコチン依存症治療アプリ」は治験、薬事承認、保険適用を経て2020年12月にCure Appより発売されました。患者は喫煙すると上昇する呼気の中の一酸化炭素濃度を、専用機器で毎日測ってアプリに記録し、服薬状況や副作用の有無を入力します。医師は患者の入力した内容を医師用アプリで確認し、その後の診察に生かします。治験では、標準禁煙治療群の9〜52週の禁煙継続率は41.5%でしたが、同アプリを活用した群は52.3%と高いものでした。同アプリはすでに100以上の医療機関が導入したとされます。また、同社では、高血圧症治療用アプリも開発し、今年5月に薬事申請を行いました。患者が自宅で記録する日々の血圧や生活習慣をもとに、個々の患者の状況に合わせた治療ガイダンスを表示し、意識や行動の変容を促すものです。治験では、未治療の高血圧患者390例を対象に行ったところ、標準的な生活習慣の改善を行った群(対照群)の12週間後の自由行動下血圧測定(ABPM)による24時間収縮機血圧のベースラインからの変化はマイナス2.5mmHgでしたが、アプリ群ではマイナス4.9mmHgと 、有意な降圧効果が認められました。両群の差である2.4mmHgの降圧は、脳心血管疾患の発症リスクを10.7%低下させると考えられています。
サスメドは、認知行動療法(CBT)によって不眠症を治療するアプリの開発を進め、100人以上の治験を実施しています。患者はスマホアプリを通じて定期的に睡眠に関するCBTのプログラムを受け取ります。対面のCBTは1回30〜60分 かけて実施されますが、それを毎日分散して行うイメージといいます。
デジタル治療用アプリについては2014年の医薬品医療機器等法が改正され、ソフトウェアを「医療機器プログラム」として扱うことが可能となりました。それに伴い、各疾患領域の患者を支援するデジタル治療用アプリの開発が加速しています。ポストコロナ時代において通院時以外での行動変容への働きかけにより治療効果を高めるデジタル治療用アプリは新たな治療の選択肢として期待されます。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。