
Specially Appointed Professor, Tokyo Medical and Dental University
Director of the M&D Data Science Center, Tokyo Medical and Dental University
Satoru Miyano
Graduated from the Department of Mathematics, Faculty of Science, Kyushu University in 1977. Doctor of Science. Professor at Kyushu University Faculty of Science in 1993, Professor at Human Genome Center, Institute of Medical Science, University of Tokyo in 1996, Director of Human Genome Center in 2014, and President of Kanagawa Cancer Center in 2015. Director of the newly established M&D Data Science Center at Tokyo Medical and Dental University in 2020. ISCB Fellow of the International Society for Computational Biology (ISCB). Recipient of the Uehara Prize in 2016 (for "Uncovering the molecular basis of cancer through cutting-edge genomics"), the Healthy Society Award (Pioneer Division) in 2019, and the Okawa Prize in 2023 (for "Whole genome analysis using supercomputers and cutting-edge research in cancer genome research").
The Act on Comprehensive and Planned Promotion of Measures to Ensure that the Public Can Receive High-Quality and Appropriate Genomic Medicine with Peace of Mind (commonly known as the Genomic Medicine Promotion Act) will come into effect in June 2023, and attention is being focused on genomic medicine, which uses individuals' genomic information to personalize treatment. We spoke to Project Professor Satoru Miyano of Tokyo Medical and Dental University, who has been leading research on the collection and utilization of medical data, including genomic information, for many years, about the present and future of genomic medicine.
The Genomic Medicine Promotion Act defines genomic medicine as "medical treatment provided to an individual based on the characteristics of the base sequence that constitutes the nucleic acid of the individual's cells or the characteristics of the function of the nucleic acid." The area in which genomic medicine is most advanced is cancer. It was revealed in the 1970s that cancer is a disease caused by genetic damage, and it has become clear that the types and number of genes that become abnormal, as well as the form of abnormality (base replacement, copy number abnormality, structural abnormality of chromosomes, etc.), vary depending on the type and progression of cancer, and that even cancer in the same organ differs from patient to patient. Molecular targeted drugs that target such genetic abnormalities have been developed, and it has become standard to examine patients' genetic abnormalities.
The cancer genetic tests currently being performed are shown in Table 1.
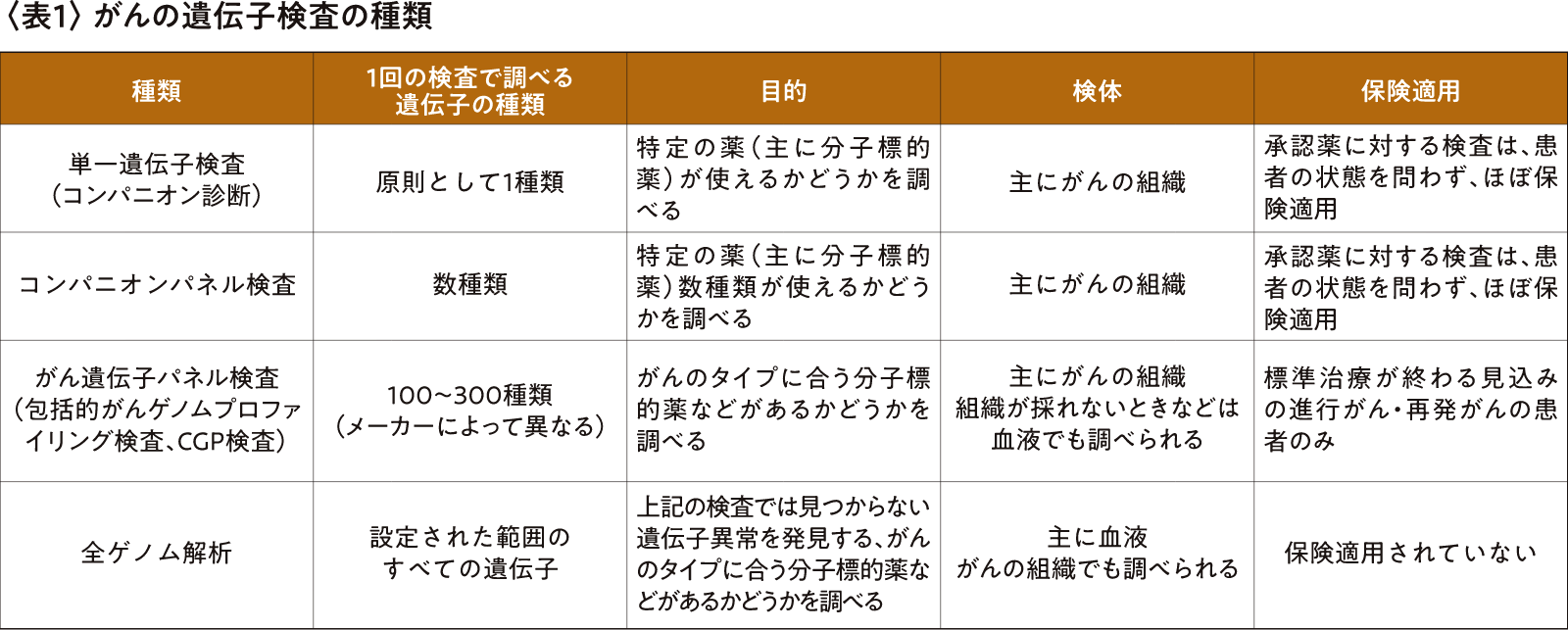
In 2019, cancer gene panel testing became covered by insurance, and the establishment of cancer genomic medical systems such as core hospitals for cancer genomic medicine made it easier to handle genetic testing, and progress was made in the training of experts to join expert panels, such as genetic oncologists and genetic counselors.
However, currently, it takes at least one month for a patient to go to a hospital where a cancer gene panel test can be performed, receive explanations and counseling, undergo the test, receive the results, and decide on a treatment plan. Insurance only covers patients with recurrent or advanced cancer who are expected to have completed standard treatment, and there is a limit of the number of times the test can be taken. Only about 10-15% of patients are able to find a suitable drug after undergoing the test, and even if a drug is found, there are patients whose health has already deteriorated and who are unable to receive treatment.
A single cancer gene panel test costs 560,000 yen (168,000 yen with a 30% self-pay), and the government is hesitant to expand the scope of insurance coverage due to the enormous medical expenses if everyone diagnosed with cancer were to undergo this test, as well as a shortage of manpower.However, I believe that requiring a panel test after a cancer diagnosis would not only improve the effectiveness of treatment, suppress the progression and recurrence of cancer, and reduce treatment and hospitalization costs, thereby not only benefiting patients but also reducing the total national medical expenses.
It is also important to point out that companion diagnostics and cancer gene panel tests examine specific types of genes, and they cannot detect genetic abnormalities that are not included in those tests.
In fact, there was a case where a patient with progressing cancer, whose cause could not be found even after cancer gene panel testing, visited the Institute of Medical Science, University of Tokyo Hospital, where whole genome analysis revealed epigenetic (DNA sequence-independent) genetic abnormalities, and an appropriate drug was found, leading to a full recovery. There was also a case where a young male patient with acute leukemia was found to have a rare type of genetic abnormality through a paper search after whole genome analysis, and the genetic abnormality disappeared after anticancer drug treatment, so it was discovered that a bone marrow transplant was not necessary and that only observation would be necessary.
The cost of whole genome analysis is falling year by year thanks to the development of gene sequencers and computers, and large infrastructure is no longer necessary due to miniaturization of equipment and data storage in the cloud. The work of expert panels, which becomes more burdensome as the number of genes to be analyzed increases, will likely be assisted by AI in the near future to improve efficiency and accuracy.
We believe that by creating a new framework for whole genome analysis in advanced medical treatment, having patients cover the costs of the testing, and incorporating whole genome analysis at cancer genome core hospitals and other institutions that are accustomed to handling cancer gene panel testing, both current and future patients will benefit.
There are already countries around the world that have adopted genomic medicine as policy and have systems in place that allow people to receive whole-genome analysis if they are diagnosed with cancer.
In Japan, an organization is scheduled to be established next year to oversee whole genome analysis for patients with cancer and intractable diseases based on the "Whole Genome Analysis Action Plan 2022"1. Furthermore, in addition to whole genome analysis, efforts are also underway to create a system that will make it easier to approve new drugs and expand the application of existing drugs so that the results can be utilized. I hope that the system will be designed not only to research genomic medicine, but also to develop new treatments and give back to patients through treatment. I myself will focus on creating a system and developing human resources that will contribute to this system.
1 Ministry of Health, Labor and Welfare "Whole Genome Analysis Action Plan 2022"
(https://www.amed.go.jp/content/000128700.pdf)