ASOURCE®NAVI
公開日:2022.09.21
新型コロナウイルスのオミクロン株に対応したワクチンの追加接種が9月20日から始まりました。従来のワクチンで4回目接種の対象となっている60歳以上の高齢者や18歳以上の基礎疾患のある人、医療従事者から始め、10月以降に対象者を広げることにしています。オミクロン株対応ワクチンの効果、副反応、接種対象・時期などについてまとめました。
オミクロン株に対応したファイザー製とモデルナ製のワクチンは、従来株(中国・武漢株)由来の成分に、第6波で広がったオミクロン株のBA.1由来の成分を混ぜたものです。BA.1に対応し、現在流行しているBA.5に対しても効果が見込まれています。
オミクロン株は従来の新型コロナウイルスから大幅に変異したため、従来のワクチンでは感染を防ぐことが難しくなっていました。
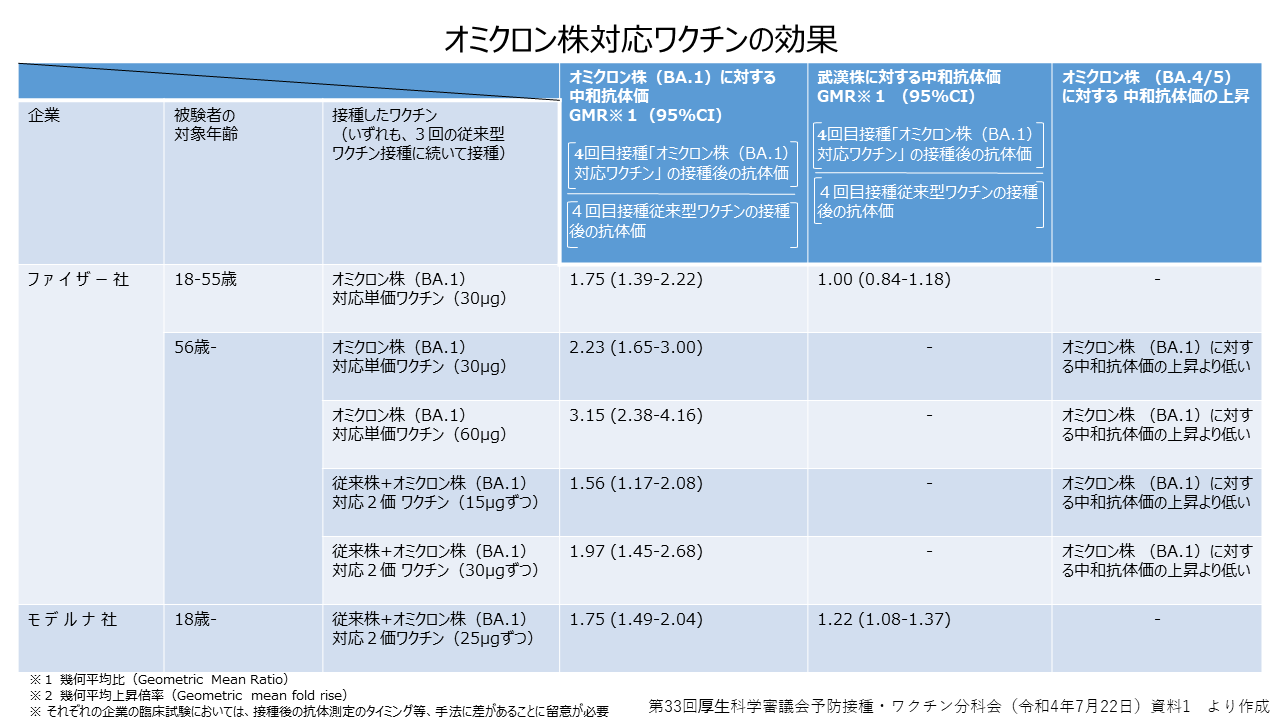
オミクロン株対応ワクチンの臨床試験結果によると、BA.1に反応して感染を予防する中和抗体は、従来ワクチンの追加接種と比較してファイザー製で1.56倍、モデルナ製で1.75倍とされ、従来型より、効果は高いことが見込まれています。中和抗体の産生量が増えれば、感染予防効果も高くなることが考えられています。また、従来ワクチンを上回る重症予防効果も期待されています。いずれのワクチンも国内で流行中のBA.5に対しても一定程度の効果があるとみられています。
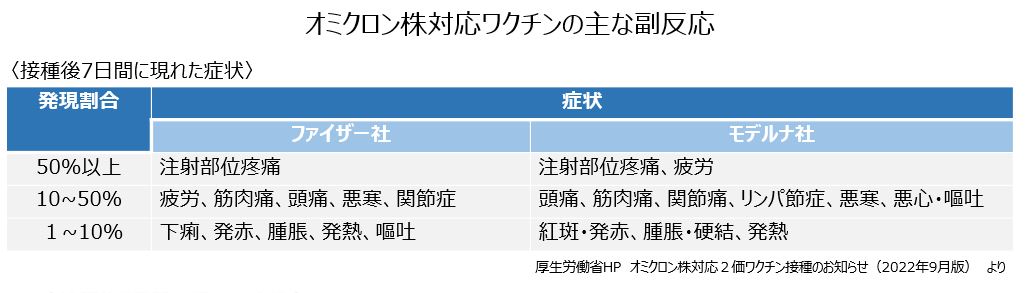
接種後の副反応については、両社とも、従来のワクチンとおおむね同じで、接種部位の痛み、だるさ、頭痛、筋肉痛、関節痛、悪寒、発熱などの症状で、軽度から中等度と報告されています。
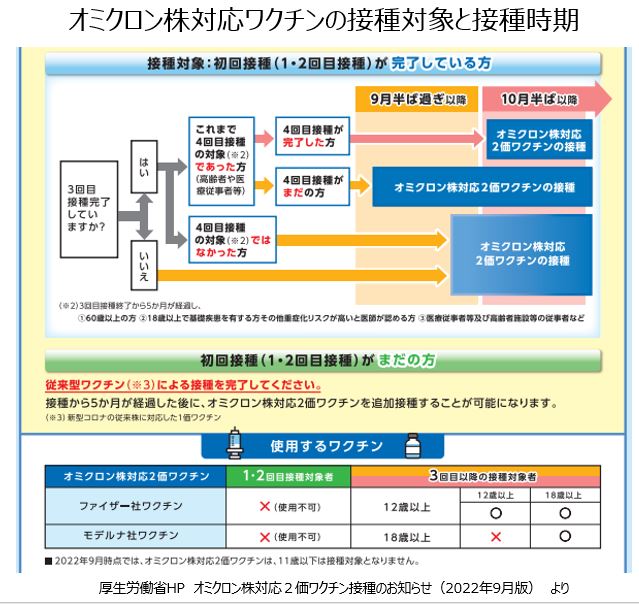
今回のワクチンは追加接種用として承認されるため、2回接種が完了した人が対象となります。予防接種法に基づく臨時接種に位置づけられ、無料で接種できます。前回の接種から5か月以上経過した人でファイザー製は12歳以上、モデルナ製は18歳以上に使用します。
従来のワクチンで4回目対象となっている60歳以上の高齢者、18歳以上の基礎疾患のある人、医療従事者は9月20日以降に優先的に接種できるようになっています。10月中旬以降は、2回接種を完了していて前回接種から5か月が経過している12歳以上の全ての人が接種できるようになります。
現在、感染の主流を占めているのは、オミクロン株の中でもBA.5ですが、ファイザーは、すでに、BA.4、BA.5に対応するワクチンの承認申請を行い、近く導入が見込まれます。このワクチンは、BA.4とBA.5に対する中和抗体が増え一定の有効性があるとされます。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。