Release date: 2021.05.24
The new coronavirus vaccine developed by Moderna in the US and AstraZeneca in the UK was approved on May 21st. The supply of rice and Pfizer products, which have already been inoculated in advance, will increase significantly, and it is expected that the inoculation speed will accelerate. The latest findings of these vaccines are combined to summarize the characteristics and efficacy.
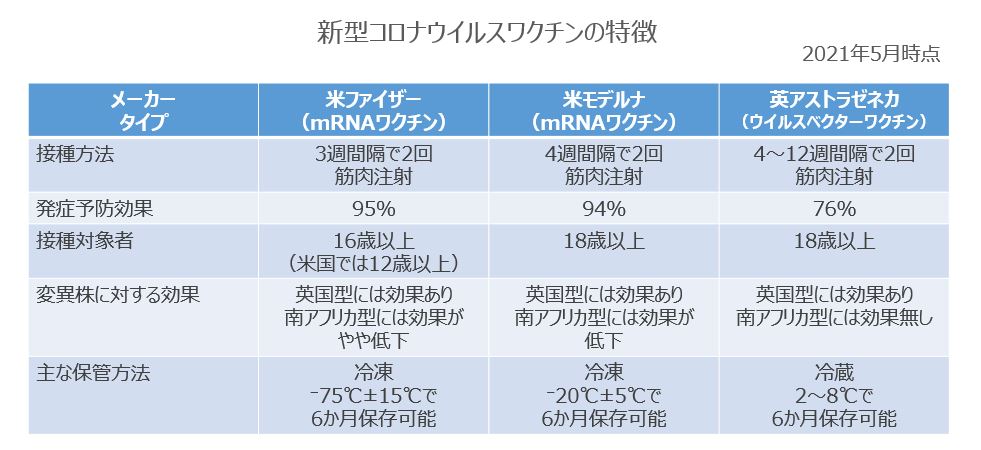
Drawing with reference to the Japanese Association for Infectious Diseases Vaccine Committee COVID-19 Vaccine Proposals (2nd Edition)
Pfizer's product was developed with the German biotechnology company Biontech and is called the mRNA vaccine. MRNA, which serves as a "design drawing" for spike proteins that serve as a foothold when coronavirus infects humans, is coated with lipids and administered into the body, and coronavirus proteins are produced in cells based on the information in the design drawings. Let me. As a result, immunity is induced and coronavirus infection is prevented.
Since mRNA is an unstable substance, it needs to be stored in ultra-low temperature freezing. Until now, storage at around -75°C (up to 6 months) has been essential, but recent findings have made it possible to store at around -20°C for up to 2 weeks. Being able to handle it in a regular drug freezer makes it easier to transport in rural areas and store in clinics. In addition, if it is taken out of the frozen state at a medical institution and not opened, it can be stored in a refrigerator at 2 to 8°C for up to 5 days (EU Pharmaceutical Regulatory Authority European Medicines Agency EMA says from 5 days to 1 month. We are issuing new guidelines to extend it). The inoculation method is intramuscular injection, and it is necessary to receive the second inoculation 3 weeks after the first inoculation. The target age for vaccination is 16 years or older, but in the United States it has expanded to 12 years or older.
The preventive effect in clinical trials is reported to be 95%. Regarding the infection prevention effect, according to the data of the research group of Kleit Health Services in Israel reported in the electronic version of The New England Journal of Medicine (NEJM) in February this year, about 600,000 vaccinated people and about non-vaccinated people. Comparing 600,000 people, it was confirmed that the infection prevention effect was about 92%. Regarding the effect of the vaccine on the mutant strain, the data of the Cornell Medicine research group in Qatar reported to NEJM in May this year showed that the experiment using the antibody of the vaccinated person at the cellular level was against the British mutant strain. After 2 weeks of the second inoculation, the effect was 89.5%, and the effect was slightly reduced to 75% for the South African mutant strain.
As a side reaction, according to the data of the US Center for Disease Control and Prevention (CDC) reported to JAMA Insights in April this year, about 1.66 million people who were vaccinated were analyzed, and those who had pain at the vaccination site were the first vaccination. About 64% after the second vaccination, about 67% after the second vaccination, about 7% after the first vaccination, about 22% after the second vaccination, and 1 for those who feel tired. It is about 29% after the second vaccination and about 48% after the second vaccination.
The Pfizer vaccine was approved on February 14, this year, and three days later, priority vaccination with healthcare professionals began. The country has a contract to receive vaccines for 97 million people by the end of the year.
The Moderna vaccine is the same mRNA vaccine as Pfizer. It is said that it can be stored in a freezer at -25°C to-15°C (up to 6 months), and at the inoculation site, it can be stored in a refrigerator at 2-8°C for 30 days. The inoculation method is intramuscular injection, and it is necessary to receive the inoculation in the 4th week after the first inoculation. The target of vaccination is 18 years old or older.
The preventive effect in clinical trials is reported to be 94%. Regarding the effect of the vaccine on the mutant strain, data from Moderna and others reported to NEJM in April this year showed no significant effect on the effect on the UK mutant strain. Serum neutralizing activity was reduced by about one-sixth for South African mutants and about one-third for Brazilian mutants.
As a side reaction, according to the CDC data reported to JAMA Insight in April this year, about 1.98 million people who were vaccinated were analyzed. About 78% after the first vaccination, about 10% after the first vaccination for those who have fever, about 38% after the second vaccination, and about 33 after the first vaccination for those who feel tired. %, About 60% after the second inoculation.
The Moderna vaccine was applied for approval in March, and the country has signed a contract to supply vaccines for 25 million people by September this year. Immediately after approval, the policy is to prioritize distribution to large-scale inoculation venues in Tokyo and Osaka for the time being, and then expand to medical institutions nationwide.
The AstraZeneca vaccine was co-developed with the University of Oxford. RNA, which serves as a "blueprint" for spike proteins that serve as a foothold when coronavirus infects humans, is administered as a carrier (vector) by incorporating it into chimpanzee adenovirus. Then, adenovirus infects human cells, spike proteins are produced in the body, immunity of the living body is induced, and antibodies are produced.
While Pfizer and Moderna require transportation and storage at ultra-low temperatures, this vaccine can be stored in a refrigerator at 2-8 ° C (up to 6 months) and is easy to handle. The inoculation method is intramuscular injection, and it is necessary to receive the second inoculation 4 to 12 weeks after the first inoculation. The target of vaccination is 18 years old or older.
The preventive effect in clinical trials is reported to be 76%. Regarding the effect of the vaccine on the mutant strain, according to the data of the research group such as Oxford University reported in the electronic version of Lancet in March this year, the II / III of about 8,500 people who received two vaccinations. After-the-fact analysis of a phase-randomized trial found that the vaccine efficacy rate was 70.4% for the UK variant.
In addition, according to data from a research group at the University of Whitwatersland in South Africa reported to NEJM in March this year, a clinical trial involving approximately 2.000 people in South Africa showed mild to moderate vaccination and non-vaccination. There was no difference in the number of patients who developed the symptoms of South Africa, and the preventive effect of the South African mutant strain was only 10.4%.
According to AstraZeneca's published adverse reaction material, the adverse reaction was inoculated after analyzing about 12,000 people who received at least one dose in four clinical trials conducted in the United Kingdom, Brazil and South Africa. Later inoculation site pain was 54.2%, fever was 33.6%, and fatigue was 53.1%.
By the end of March this year, about 20.2 million doses had been inoculated in the UK, 79 cases of thrombosis were reported, and 19 of them died, according to the UK Medicines and Healthcare Products Regulatory Agency. It is often seen in young women, and it is said that most of them have cerebral vein thrombosis within 2 weeks after inoculation. By early April this year, EMA reported 222 out of 34 million cases of cerebral venous thrombosis, most of which occurred in women under the age of 60. Under these circumstances, Denmark and Norway have stopped vaccination, and in Germany, there are movements to limit vaccination to those over 60 years old.
The Ministry of Health, Labor and Welfare has decided to exclude AstraZeneca products from public expense vaccination for the time being, and with the cooperation of related academic societies, prepare a manual for dealing with thrombosis and consider vaccination targets such as age restrictions. ..
The AstraZeneca vaccine was filed for approval in February of this year, and the country has signed a contract to supply the vaccine for 60 million people.
MEDIUS Group is developing a business centered on the sale of medical equipment. We (Medical + us) involved in medical care also want to play the role of an information source (Media) that delivers useful information for the medical field and people's healthy tomorrow.