
Release date: 2022.03.17
As the use of foreign-made new coronavirus vaccines progresses, it is expected that a vaccine by a Japanese pharmaceutical manufacturer, which has been independently developed for its convenience and safety, will finally be put into practical use.
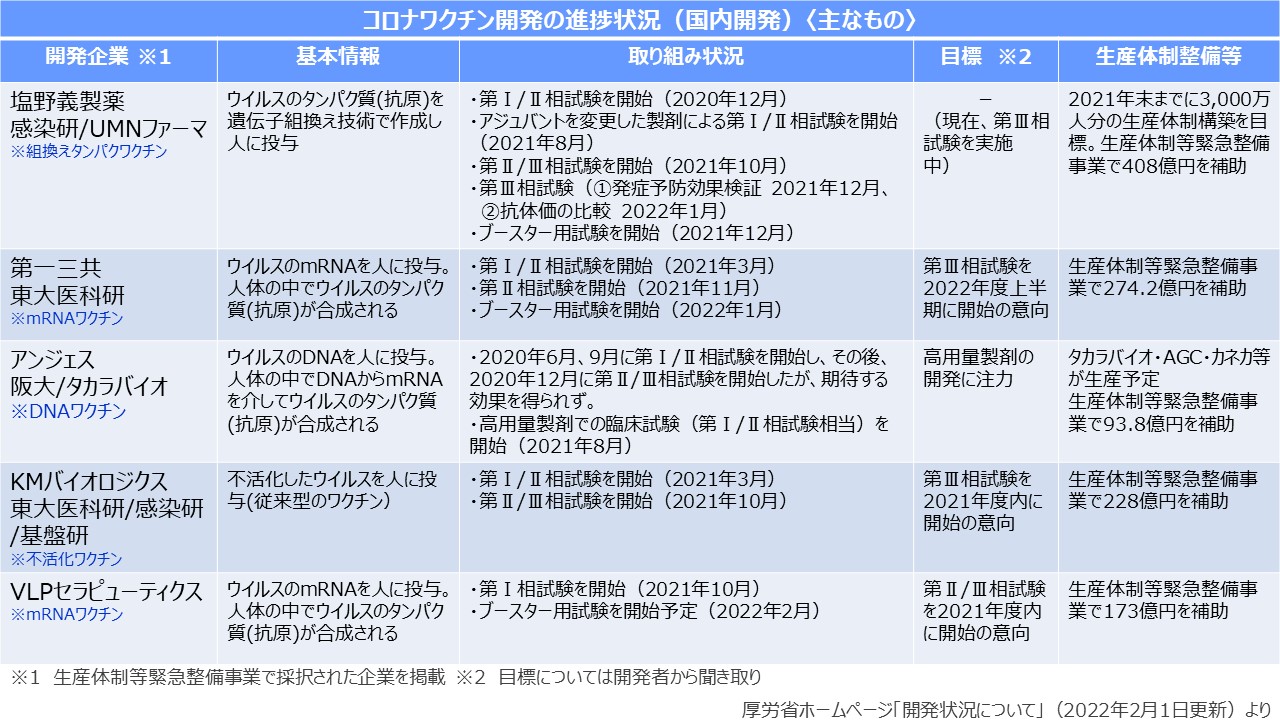
Shionogi Pharmaceutical Co., Ltd., which is ahead of the domestic corona vaccine development, is prioritizing clinical trials that are supposed to be used for booster vaccinations rather than clinical trials for unvaccinated people. This vaccine is a recombinant protein vaccine that creates the base substance (antigen protein) of the vaccine from the genetic information of the virus, and it is already used in vaccines such as herpes zoster. The recently published interim report of the phase 2/3 clinical trial of booster vaccination showed that the efficacy was the same as that of Pfizer, and side effects such as fever and headache were low.
In this clinical trial, about 200 adults in Japan who have been inoculated with two doses of Pfizer's vaccine for more than 6 months have been vaccinated with a vaccine under development as a booster and a group with a Pfizer vaccine. and compared efficacy and safety. As a result, the vaccine group under development had 1.17 times the neutralizing antibody titer approximately one month after vaccination compared to the Pfizer vaccine group, confirming that they are equally effective. . In addition, the incidence of side reactions was higher with the vaccine under development than with Pfizer (Shionogi: 38.8%, Pfizer: 59.2%), headache (Shionogi: 25.2%, Pfizer: 41.7%), It was shown to be low in fatigue (Shionogi: 43.7%, Pfizer: 53.4%). Pain and muscle pain at the injection site were also less than the same with Shionogi as compared with Pfizer. Based on this result, the company will proceed with preparations for approval application, aiming to start supply after May this year. On the other hand, the final-stage clinical trial for unvaccinated people is targeted at about 50,000 people in Southeast Asian countries such as Vietnam, where 80% of the population in Japan has been vaccinated (completed two doses). is considered to be in progress.
Daiichi Sankyo, which is developing a vaccine using the same messenger RNA (mRNA) as Pfizer, which has already started inoculation to the general public, has changed its conventional development policy and will precede clinical trials for additional vaccinations from this year. We are rushing to supply it by the end of the year. As a result, we decided to postpone the final-stage clinical trial for unvaccinated people. Pfizer's vaccines require ultra-low-temperature storage, but Daiichi Sankyo's goal is to create vaccines that can be refrigerated. KM Biologics is also proceeding with clinical trials of booster vaccinations in the form of physician-led initiatives. The company is developing an inactivated vaccine that has lost the infectivity of the same virus as the influenza vaccine, and the company says that its safety is guaranteed from its long-term use record.
AnGes was one of the first to develop a vaccine that administers plasmid DNA containing viral genetic information. Commencement is expected around 2023. VLP Therapeutics is developing a new type of corona vaccine that can be expected to be effective with a small amount of inoculation, and started early-stage clinical trials in October last year. This vaccine is a messenger RNA (mRNA) called a self-replicating type that increases in the body after vaccination, and is characterized by a 1/10 to 1/100 dose compared to Pfizer and Moderna. is expected to be reduced. On the other hand, Mitsubishi Tanabe Pharma is proceeding with clinical trials of a plant-based vaccine developed by its Canadian subsidiary (Medicago) for those who have not been vaccinated in Japan, and aims to apply for approval by this fall. This vaccine is a vaccine that incorporates virus genes into plant leaves and extracts vaccine components. The frequency of fever after two doses is less than 10%, and a clinical trial for booster vaccination is also under consideration.
It is said that there is a difference between Europe and the United States, which emphasize efficacy in the research and development of pharmaceuticals, and Japan, which emphasizes safety.
MEDIUS Group is developing a business centered on the sale of medical equipment. We (Medical + us) involved in medical care also want to play the role of an information source (Media) that delivers useful information for the medical field and people's healthy tomorrow.